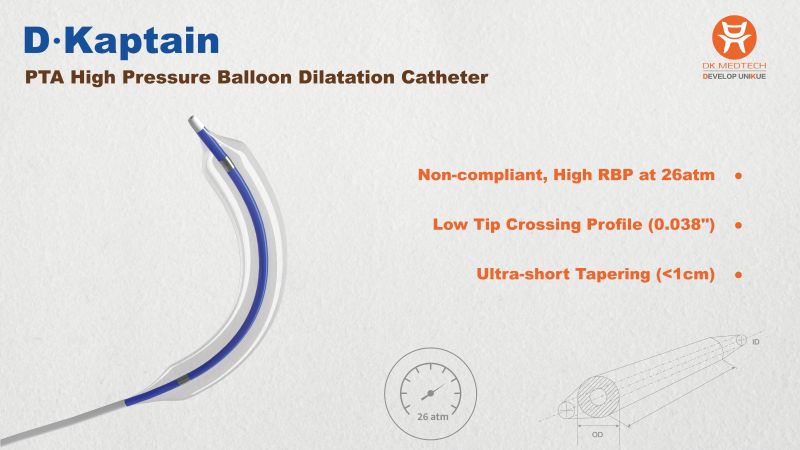
Breakthrough Technologies Address Dialysis Clinical Challenges
Press summary: DK Medtech and Mozarc Medical forge strategic partnership to elevate vascular access care for U.S. dialysis patients with innovative balloon technologies Suzhou/Minneapolis, June 25, 2025 — DK Medical Technology (Suzhou) Co., Ltd. (DK Medtech), a leading Chinese innovator in interventional balloon technologies, and Mozarc Medical, a global pioneer in kidney