Suzhou, China — Recently, DK Medtech announced a significant milestone: its independently developed DK Score™ Coronary Scoring Balloon Dilation Catheter has successfully obtained the CE MDR certification (CE: M.2025.MDR.1062). This achievement signifies that the product has officially gained market access qualifications in the EU and other countries and regions that recognize this certification.

DK Score™ Coronary Scoring Balloon Catheter
Innovative Design, Leading the New Trend of Interventional Treatment
The DK Score™ Coronary Scoring Balloon is an innovative product of DK Medtech, featuring a unique patented design that offers excellent crossability and directional dilation capability. The scoring elements of this product are made using the “triangular nitinol coil” technology, with a cross-section in the shape of an equilateral triangle. Three scoring elements are distributed at 120° intervals and securely fixed on the balloon working segment. This design ensures that during the dilation of highly resistant stenotic lesions, the scoring elements will not slip. The triangular design can efficiently embed into the vascular intima, coupled with a rated burst pressure of 20 atm, providing the local high pressure required for directional dilation. Moreover, for highly stenotic tortuous lesions, the patented nitinol coil design achieves strong longitudinal strength while maintaining axial flexibility, significantly improving the balloon’s crossability.

Clinical Validation Highlights Outstanding Performance
In a pre-market clinical study (ClinicalTrials.gov ID: NCT05250193), led by Professor Zhou Yujie from Beijing Anzhen Hospital, the largest sample size (n=200) RCT trial of pressure-focused balloons in China showed the following outstanding results:
- the acute lumen gain for the DK Score™ group was 1.12mm, while the control group had an acute lumen gain of 0.95mm (p=0.005), showing a statistically significant difference.
- The device procedural success rate for the DK Score™ group was 96.0%, compared to 89.7% for the control group.
These excellent data fully demonstrate the outstanding performance and clinical value of the DK Score™ Coronary Scoring Balloon.

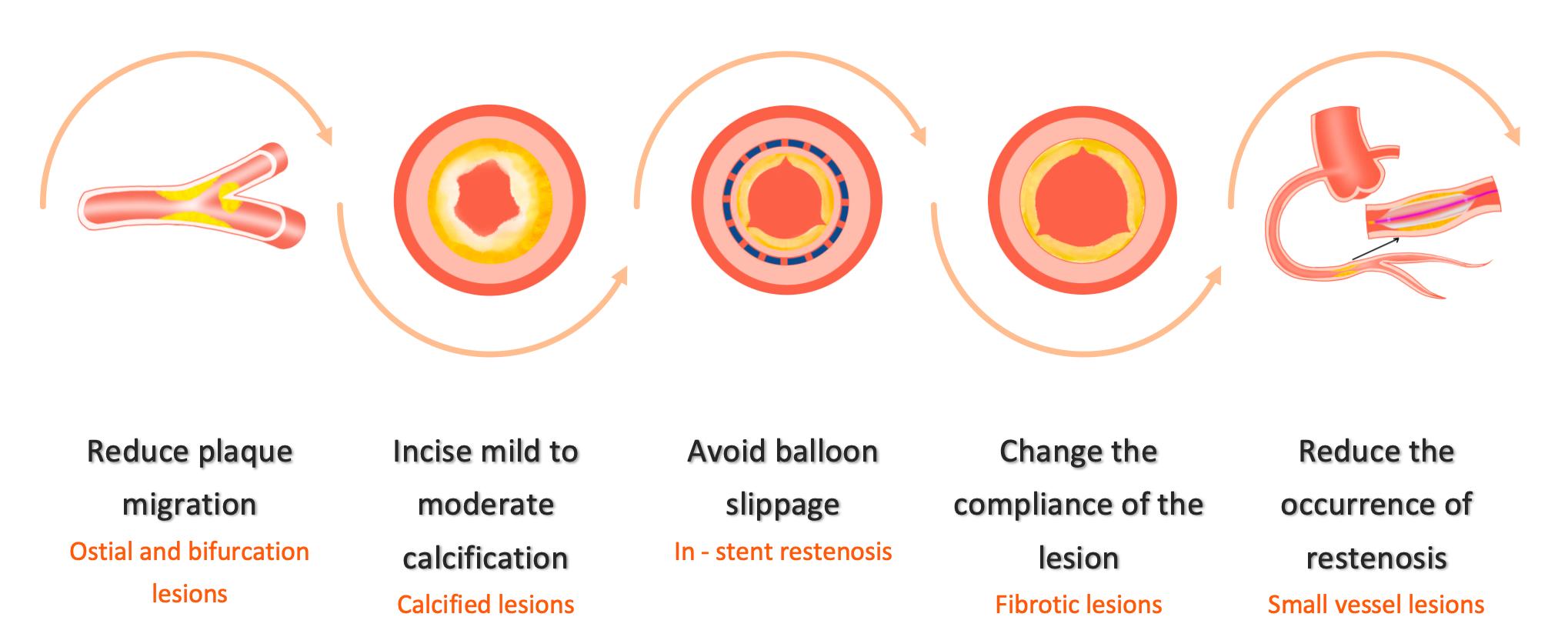
Five Major Complex Lesions, One Solution
International Journey to Serve Global Patients
The acquisition of the overseas CE certification is not only an affirmation of the technical strength of the DK Score™ Coronary Scoring Balloon but also a significant milestone in DK Medtech’s international strategy. The CE certification is a mandatory certification for medical devices to be sold in the EU market. Obtaining this certification means that the product complies with the requirements of EU regulations and harmonized standards, and has the qualification to be sold in the EU and other countries and regions that recognize this certification. In the future, DK Medtech will continue to adhere to the concept of independent innovation, continuously develop and launch more high-quality medical device products, and provide better medical services for patients worldwide.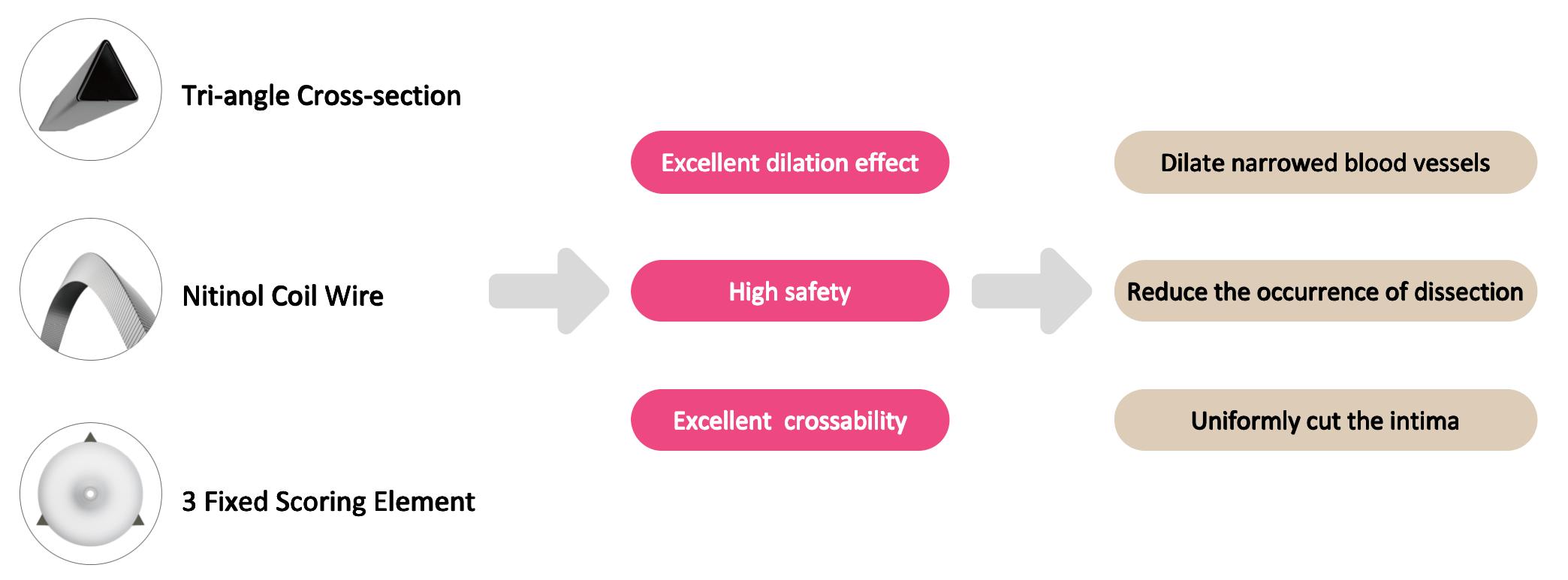
About Us
Since its establishment in 2015, DK Medtech has always been committed to innovation-driven development. It has now become a leading domestic full – category interventional balloon innovation enterprise. It has researched and developed more than ten types of balloons, including standard balloons, high – pressure balloons, scoring balloons, and drug – coated scoring balloons. Its product line covers the needs of almost all vascular interventional fields, such as cardiovascular interventional, peripheral interventional, and neuro interventional fields.
Through its independently developed directional scoring technology, which is used in coronary scoring balloons, peripheral scoring balloons, and drug – coated scoring balloons, the company has achieved significant clinical benefits compared to the imported control group, according to the published pre – market RCT studies. It has also achieved excellent results and market positions in niche markets. In addition, DK Medtech has signed multiple strategic cooperation agreements with well – known foreign enterprises such as Medtronic and Asahi Intecc.


