Suzhou, China — Recently, DK Medtech (Suzhou) Co., Ltd. (hereinafter referred to as “DK Medtech”) announced that its independently developed DKutting® Scoring Balloon Dilation Catheter has successfully obtained the EU CE MDR certification (CE: M.2025.MDR.1065). This certification marks a significant milestone, enabling the product to enter the EU market and other regions that recognize this certification, and highlights the international recognition of Chinese medical device innovation and product quality.

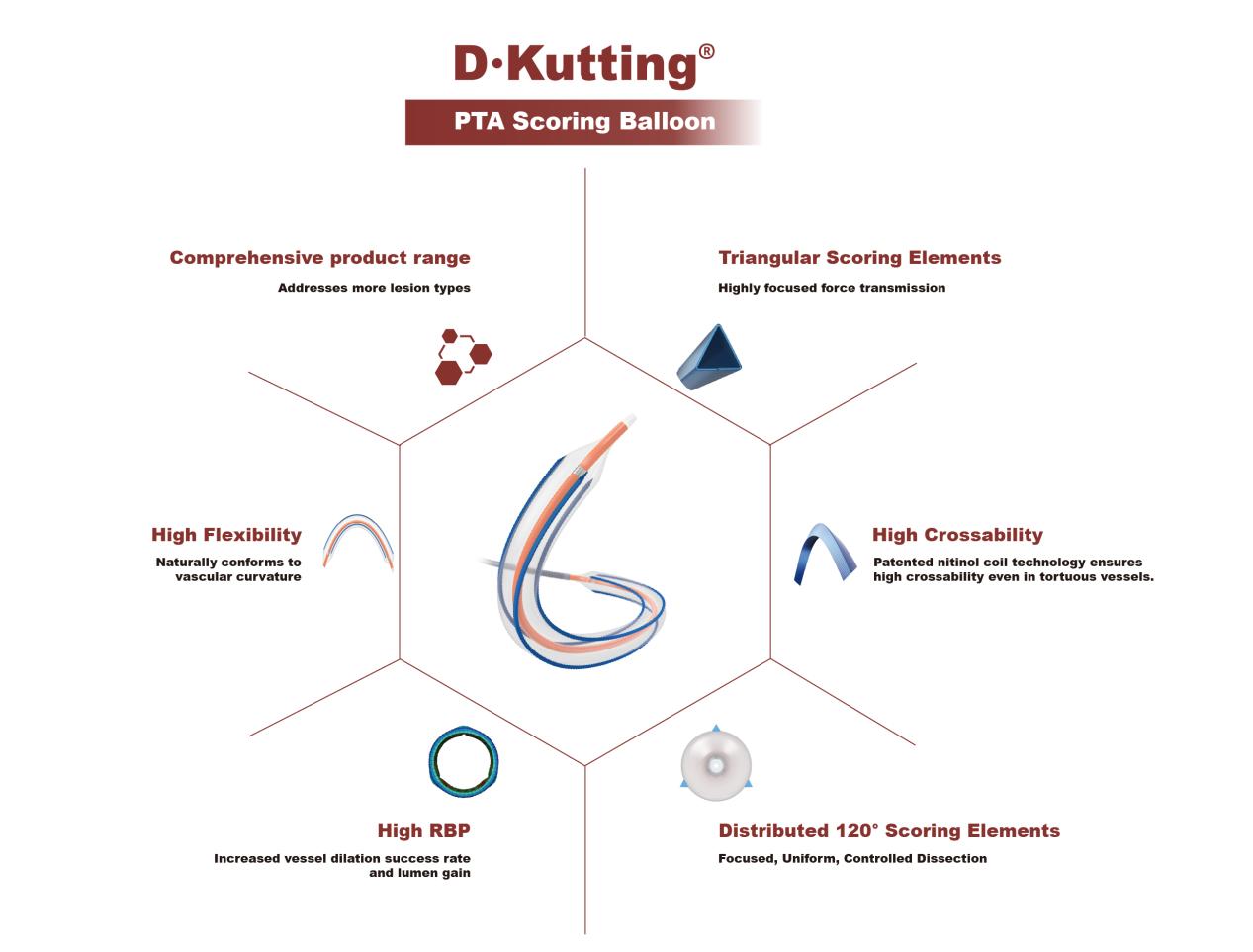
Innovative Design Leads the New Trend in Interventional Treatment
The DKutting® High-Pressure Scoring Balloon features a proprietary patented design (CN201810478242.X) with excellent crossability, uniform dilation, and high burst pressure. Since its approval by the NMPA in November 2022, it has been widely praised in peripheral vascular disease and hemodialysis access fields.
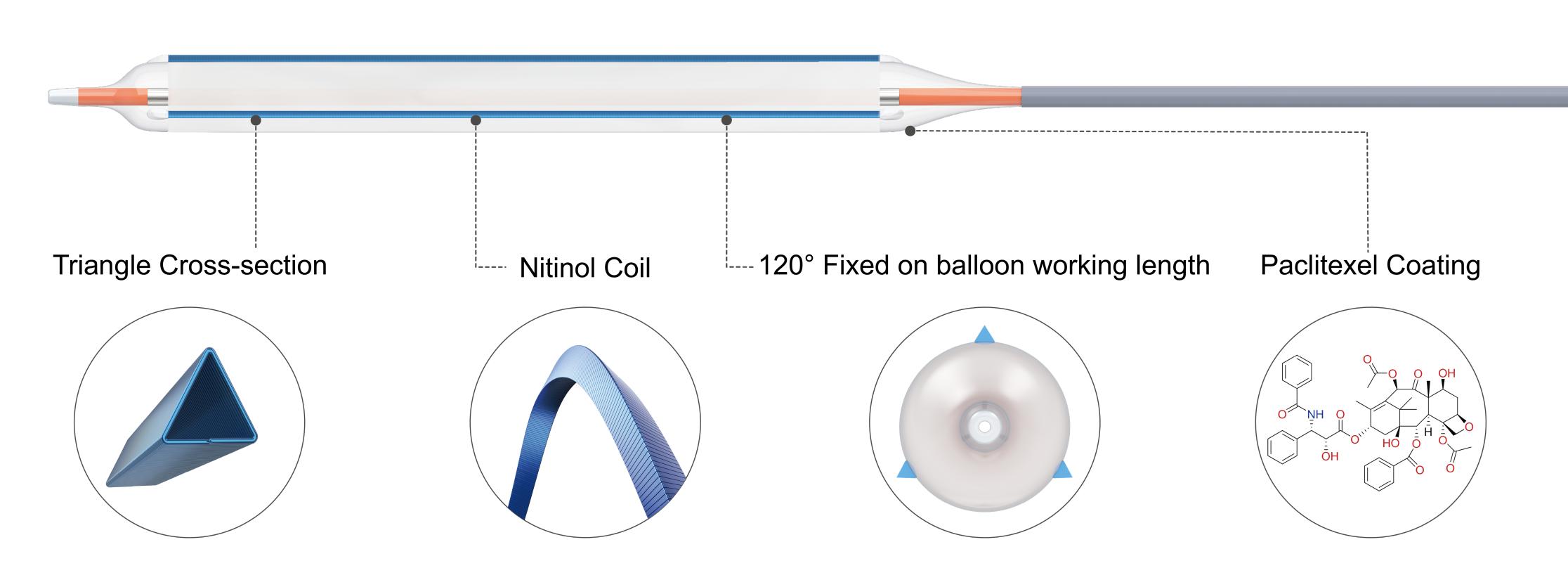
The innovative “triangular nitinol coil” scoring elements, with a cross-section in the shape of an equilateral triangle, are distributed at 120° intervals and fixed on the balloon working segment. This design prevents slippage during high-resistance stenotic lesion dilation, efficiently embeds into the vascular intima, and provides local high pressure with a rated burst pressure of 20 atm. The patented nitinol coil design also enhances crossability in highly stenotic tortuous lesions by combining longitudinal strength with axial flexibility.
Clinical Validation Highlights Outstanding Performance
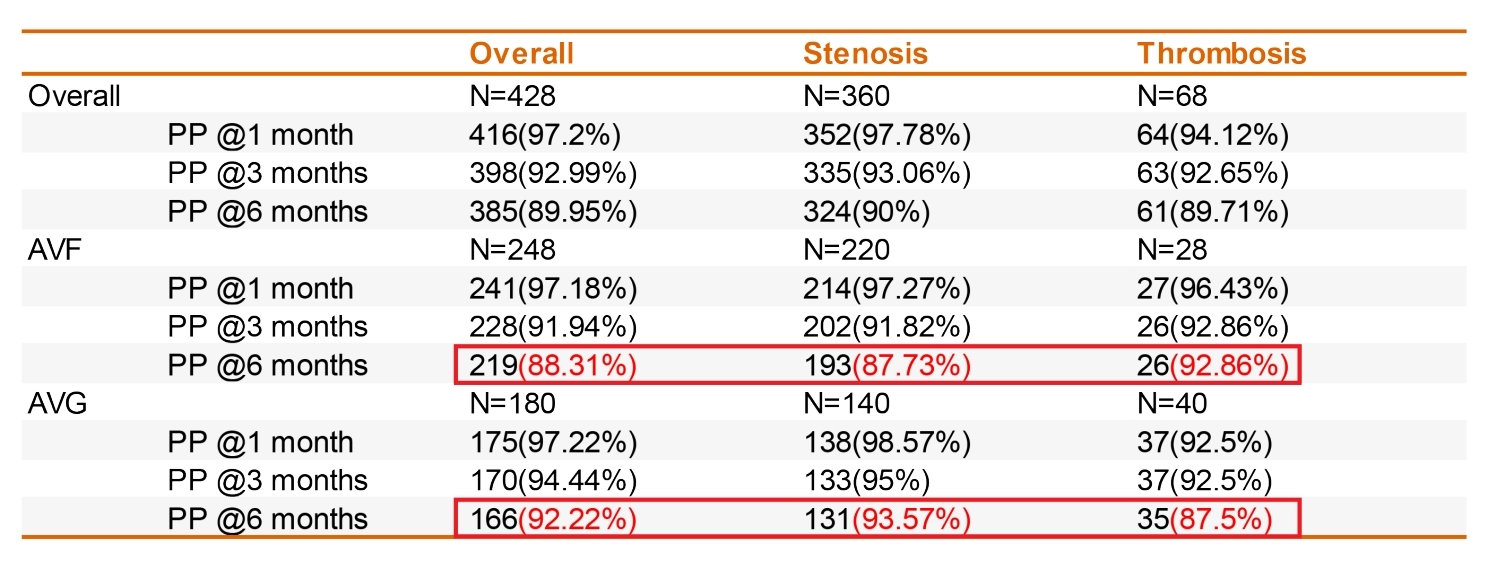
From 2023 to 2024, Beijing Haidian Hospital treated 428 cases with the DKutting® High-Pressure Scoring Balloon, including 248 arteriovenous fistulas (AVF) and 180 artificial vascular fistulas (AVG). The results demonstrated excellent dilation efficacy and safety:
– The AVF group achieved an impressive 6-month primary patency rate of 88.31%, with 87.73% in the stenosis subgroup and 92.86% in the thrombosis subgroup.
– The AVG group showed even better results, with a 6-month primary patency rate of 92.22%, 93.57% in the stenosis subgroup, and 87.5% in the thrombosis subgroup.
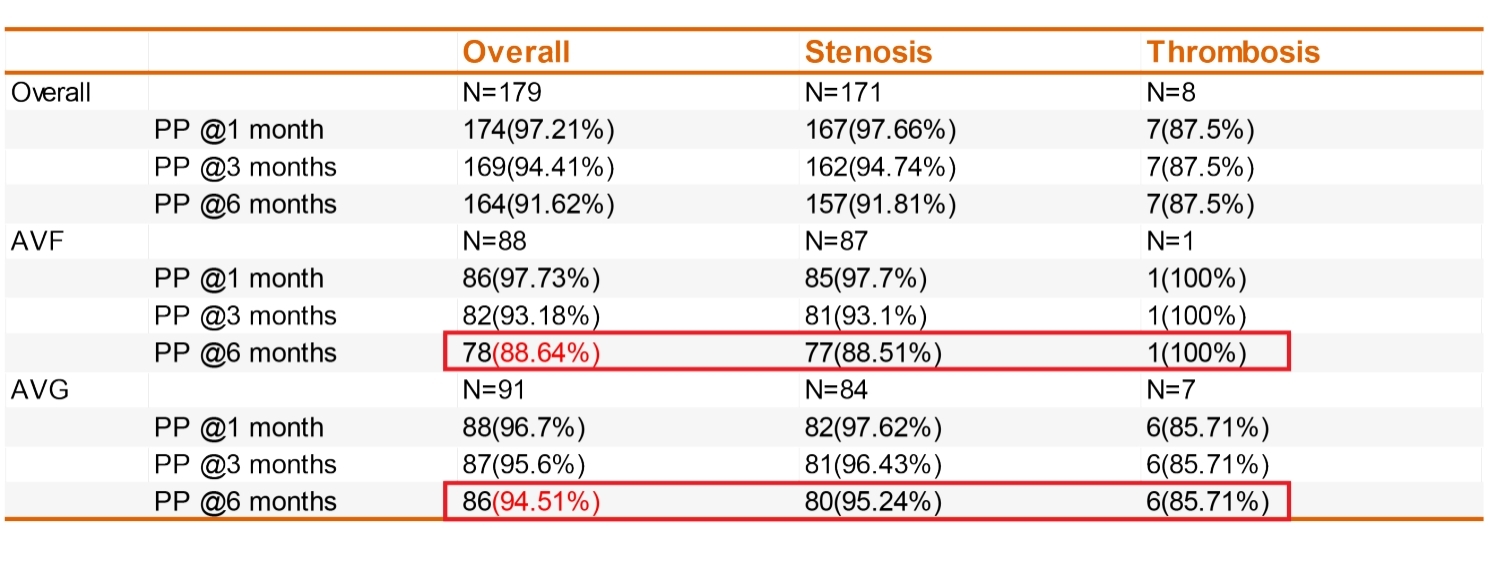
– For lesions treated with a single DKutting® balloon, the 6-month primary patency rates were 88.64% for AVF and 94.51% for AVG.
The mechanism of uniform intimal tearing minimizes vascular damage during dilation (confirmed by ultrasound and OCT), leading to higher postoperative vascular patency rates compared to other balloons.
International Journey to Serve Global Patients
The CE certification for the DKutting® High-Pressure Scoring Balloon Dilation Catheter is a testament to its technical strength and a key milestone in DK Medtech’s international strategy. This certification confirms compliance with EU regulations and standards, enabling sales in the EU and other recognized regions.
Looking ahead, DK Medtech will continue to innovate, developing and launching high-quality medical device products to provide better healthcare services worldwide.
About DK Medtech
Since its founding in 2015, DK Medtech has been committed to innovation, becoming a leading domestic full-category interventional balloon innovation enterprise. It offers over ten balloon types, including standard, high-pressure, scoring, and drug-coated scoring balloons, covering cardiovascular, peripheral, and neurovascular interventions.
With its proprietary directional scoring technology, DK Medtech’s products outperform imported counterparts in clinical benefits, as shown in pre-market RCT studies. The company has established strategic partnerships with leading international firms like Medtronic and Asahi Intecc.